Utilization of protocols to lower daily aspirin dose before surgical procedures for patients with aspirin-exacerbated respiratory disease
Rose C Corcoran, Laura B Bailey, Alyson N Brown, Kathleen M Buchheit, Jillian C Bensko, Tanya M Laidlaw
Aspirin-lowering and ibuprofen-bridging protocols are safe and effective for patients with aspirin-exacerbated respiratory disease to decrease aspirin dosing prior to elective surgical procedures, but were only utilized in half of surveyed participants, suggesting need for increased awareness. See our BWH aspirin-lowering protocol.
Efficacy of various dosing frequencies of dupilumab in patients with AERD
Alyson N. Brown, Tanya M. Laidlaw, Kathleen M. Buchheit, Jillian C. Bensko, Rose C. Corcoran, Laura B. Bailey
This study found that 32% of AERD patients on dupilumab for asthma or nasal polyposis had attempted a dosing interval other than every 2 weeks. Of those, 68% chose to continue with less frequent injections, most commonly every 3 weeks, without reduction in their perceived clinical benefit. Our data highlight the potential for patients with AERD to reduce their dupilumab dosing frequency in the future and the need for provider education about this option.
Consequences of NSAID allergy on pain control options for patients with AERD
Laura B. Bailey, Alyson N. Brown, Rose C. Corcoran, Jillian C. Bensko, Kathleen M. Buchheit, Tanya M. Laidlaw
This study demonstrates that celecoxib offers effective pain management for patients with AERD, and that non-selective NSAIDs (like ibuprofen and Naprosyn) are effective and safe for most AERD patients to take after aspirin desensitization.
We found that there is a high rate of narcotic overprescribing for patients with AERD, suggesting that reported NSAID allergies predispose our patients to the use of stronger and potentially less safe pain medications. Interestingly, despite multiple studies pointing to celecoxib as an appropriate option for many patients with AERD, narcotics were more often prescribed for pain in place of a non-selective NSAID than was celecoxib. Additional education of our pain-management colleagues regarding the safety and efficacy of celecoxib in patients with AERD may help avoid unnecessary opioid over prescribing.
Absolute eosinophil counts in aspirin-exacerbated respiratory disease are distinctly elevated and correlate inversely with respiratory function
Andrew D Supron, Victor Omilabu, Laura Bailey, Kathleen M Buchheit, Tanya M Laidlaw
This study describes the distribution and extent of blood eosinophilia in patients with AERD and assesses for correlations between absolute eosinophil count and various metrics of disease severity. In 225 patients with AERD, the absolute eosinophil count ranged from 10-4400 cells/µL, with a median 490 cells/µL, and with 78% of patients having a baseline eosinophil count >250 cells/µL, and 46% >500 cells/µL. Absolute eosinophil count was inversely correlated with lung function (FEV1 % predicted), and AERD patients with an eosinophil count >500 cells/µL had significantly lower FEV1 readings than those with eosinophil counts ≤500 cells/µL. In summary, patients with AERD have a marked eosinophilia that is even more pronounced than that of their aspirin-tolerant counterparts, and that this eosinophilia is predictive of a patient’s respiratory function via its inverse correlation with FEV1.
Validation of a questionnaire assessing smell loss in an international aspirin-exacerbated respiratory disease population
Marie Lundberg, Rie Maurer, Raffi Tchekmedyian, Jyotsna Mullur, Jillian C. Bensko, Kathleen M. Buchheit, Tanya M. Laidlaw
This study examined the validation of the questionnaires used for a prior study from our group that examined how loss of smell impacted the mental health and quality of life of patients with AERD. We determined that this new Consequences of Smell Loss (COSL) questionnaire can be used as a brief, valid, reliable tool that can effectively screen for a high burden of smell loss in patients with AERD and other patient populations where smell and olfaction are affected.
Nasal polyp antibody secreting cells display proliferation signature in aspirin-exacerbated respiratory disease
Aaqib Sohail , Jonathan Hacker, Tessa Ryan, Alanna McGill , Regan Bergmark, Neil Bhattacharyya , Stella E Lee, Alice Maxfield, Rachel Roditi , Amélie M Julé, Alec Griffith, James Lederer, Tanya M Laidlaw, Kathleen M Buchheit
Nasal polyp tissue was collected from patients with AERD and sinus disease, to explore differences in the transcriptomic profile, activation markers, and IL-5Rα expression and function of the nasal tissue plasma cells and antibody-secreting cells. It was found that nasal polyp tissue antibody secreting cells from patients with AERD express higher levels of functional IL-5Rα and increased markers associated with cell cycling and proliferation compared to those from aspirin-tolerant nasal polyp patients. This may be clinically relevant in terms of disease severity and recurrence of nasal polyposis.
Optimizing cryopreservation of nasal polyp tissue for cellular functional studies and single-cell RNA sequencing
Aaqib Sohail, Carolyn H. Baloh, Jonathan Hacker, Laura Cho, Tessa Ryan, Regan W. Bergmark , Stella E. Lee, Alice Maxfield , Rachel Roditi, Daniel F. Dwyer, Kathleen M. Buchheit, Tanya M. Laidlaw
Nasal polyp tissue can be cryopreserved as whole tissue chunks or as digested cell suspensions, and this study investigated which technique would be best for preserving nasal polyp tissue for future analyses. We determined that both methods work quite well. However, mast cell numbers were reduced in samples cryopreserved as whole tissue chunks and thawed epithelial cells had reduced proliferation rates when preserved as dissociated cell suspensions. Therefore, the right cryopreservation method to choose may depend on the goals and cell-type focus of the project and on the planned future uses.
Co-treatment of non-steroidal anti-inflammatory drug-exacerbated respiratory disease with dupilumab and aspirin therapy after desensitization
Kathleen M. Buchheit, Jonathan Hacker, Rie Maurer, Alanna McGill, Tessa Ryan, Jillian C. Bensko, Tanya M. Laidlaw
In this study, we evaluated 22 patients with AERD who were treated with dupilumab for 3 months, and 8 of those patients had also undergone aspirin desensitization prior to the start of dupilumab and continued on their high-dose daily aspirin throughout the study. We found that treatment with dupilumab in patients who were also on daily aspirin therapy led to a reduction in sinonasal eosinophilic inflammation. We did not see a reduction in sinonasal eosinophilic inflammation in the patients who were only on dupilumab without the additional daily aspirin therapy. Conversely, we saw that patients who were only on the dupilumab did develop a significant increase in the eosinophil levels in their blood, but for the patients who were on daily aspirin therapy and then started dupilumab treatment, there was no significant change in their blood eosinophil levels after starting dupilumab. Both daily aspirin therapy after aspirin desensitization, and dupilumab therapy, have been important treatments for patients with AERD, and further studies may be needed to fully understand how these two treatment options work together
Trial of thromboxane receptor inhibition with ifetroban: TP receptors regulate eicosanoid homeostasis in aspirin-exacerbated respiratory disease
Tanya M. Laidlaw, MD,a,b Kathleen M. Buchheit, MD,a,b Katherine N. Cahill, MD,c ,Jonathan Hacker, BA,b Laura Cho, BA,b, Jing Cui, PhD,a,d Chunli Feng, MD,b Chongjia C. Chen, MD,a,b Meghan Le, MS,e Elliot Israel, MD,a,b,e and Joshua A. Boyce, MDa,b Boston, Mass, and Nashville, Tenn
This clinical trial compared one month of treatment with ifetroban (a medication that blocks the thromboxane A2 receptor) to placebo in patients with AERD, to see if the ifetroban treatment decreased the severity of aspirin-induced reactions. Unfortunately, there was a small signal that actually the treatment with ifetroban actually worsened patients’ reactions to aspirin. This was unexpected, and to further understand why, additional studies were done, that showed that ifetroban inhibited the production of prostaglandin E2 and increased the levels of cysteinyl leukotrienes, which likely explains why our patients had a negative response to it.
Biomarkers and mechanisms of tolerance induction in food allergic patients drive new therapeutic approaches
Baloh CH, Huffaker MF, Laidlaw T. Biomarkers and mechanisms of tolerance induction in food allergic patients drive new therapeutic approaches. Front Immunol. 2022 Oct 3;13:972103. doi: 10.3389/fimmu.2022.972103. PMID: 36263023; PMCID: PMC9574092.
Immunotherapy for food-allergic patients has been effective in inducing desensitization in some populations, but long-term tolerance has remained an elusive target. A challenge facing our field is how to differentiate immune markers that are impacted by immunotherapy from those that are critical biomarkers of tolerance. Data from recent clinical trials have identified several biomarkers and mechanisms for achieving tolerance. These biomarkers include younger age, lower food-specific IgE, lower food component-specific IgE, specific linear epitope profiles, and subsets of food-specific CD4+ T cells. Additional biomarkers under investigation for their relevance in tolerance induction include TCR repertoires, gastrointestinal and skin microbiome, and local tissue immunity. This review highlights recent advances in understanding biomarkers and mechanisms of tolerance induction in food immunotherapy and how these are influencing clinical trial development.
Inhibiting the type 2 inflammatory pathway with dupilumab is associated with an increase in interleukin-4 and interleukin-18 production
Morris J, Olonisakin TF, Moore JA, Phan B, Parker DM, Uribe BA, Barel SJ, Bowers EMR, Buchheit KM, Laidlaw TM, Lee SE. Inhibiting the type 2 inflammatory pathway with dupilumab is associated with an increase in interleukin-4 and interleukin-18 production. Int Forum Allergy Rhinol. 2022 Oct;12(10):1313-1316. doi: 10.1002/alr.23003. Epub 2022 Apr 29. PMID: 35394682.
Given the widespread expression of IL-4Rα across effector cells in the respiratory tract, and the potential immune consequences that global inhibition of IL-4Rα may have, our goal with this study, led by Dr. Stella Lee, was to understand how the local inflammatory milieu of patients with CRSwNP changes after treatment with dupilumab. We observed a dupilumab-induced increased level of the cytokines IL-4 and IL-18 into the nasal secretions, as well as a trend toward increased CXCL8/IL-8. These may suggest a shift toward Th17/type 3 immune response, which may be indicative of a type 1/type 3 “escape” with inhibition of IL-4Rα. It has not yet been fully investigated how the local inflammatory environment changes in CRSwNP patients treated with dupilumab. Although exploratory—and thus limited in the power of our statistical analysis—these preliminary data reinforce the need for further exploration of the consequences of inhibiting the type 2 inflammatory pathway.
Updates on immune mechanisms in aspirin-exacerbated
respiratory disease
Laidlaw TM, Boyce JA. Updates on immune mechanisms in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2023 Feb;151(2):301-309. doi: 10.1016/j.jaci.2022.08.021. Epub 2022 Sep 30. PMID: 36184313; PMCID: PMC9905222.
This review covers novel insights into the immunopathogenesis of AERD from each of these lines of research, including the roles of lipid mediators, effector cell populations, and inflammatory cytokines, discusses unanswered questions regarding cause and pathogenesis, and considers potential future therapeutic options.
Aspirin desensitization and biologics in aspirin-exacerbated respiratory disease: Efficacy, tolerability, and patient experience
Mullur J, Steger CM, Gakpo D, Bensko JC, Maurer R, Laidlaw TM, Buchheit KM Ann Allergy Asthma Immunol. 2022 May;128(5):575-582. doi: 10.1016/j.anai.2022.01.043. PMID: 35131410; PMCID: PMC9058196.
In this study we surveyed 98 patients with AERD in the Brigham and Women’s Hospital AERD registry. Patients completed an online questionnaire describing their medication history and treatment experience. A total of 52 (53.0%) patients reported a history of use of one or more respiratory biologics (omalizumab, mepolizumab, reslizumab, benralizumab, or dupilumab), and 84 (85.7%) reported undergoing aspirin desensitization. There were 24 patients (24.4%) who reported concurrent use of a biologic and daily aspirin therapy. Compared with those on daily aspirin therapy alone, patients taking a biologic and aspirin therapy concurrently were less likely to report that aspirin was effective for their AERD symptoms. Whereas patients reported varying efficacy with biologics, dupilumab had the highest odds of patients reporting it worked “very well”. We conclude that biologics are emerging as a treatment option for AERD and are generally well tolerated. Biologic efficacy in AERD is variable by agent, though most patients taking dupilumab found it to be effective. Patients on a biologic in conjunction with daily aspirin therapy may represent a more severe subset of AERD for which daily aspirin therapy alone is insufficient.
Rapid and sustained effect of dupilumab on clinical and mechanistic outcomes in aspirin-exacerbated respiratory disease
Buchheit KM, Sohail A, Hacker J, Maurer R, Gakpo D, Bensko JC, Taliaferro F, Ordovas-Montanes J, Laidlaw TM. J Allergy Clin Immunol. 2022 Aug;150(2):415-424. doi: 10.1016/j.jaci.2022.04.007. PMID: 35460728; PMCID: PMC9378638.
This study followed 22 patients with AERD who were treated with dupilumab for 3 months. Participants had rapid improvement in their symptoms, including sense of smell, sinonasal symptoms, and lung function after just 1 month of dupilumab, which were sustained after 3 months of dupilumab. Baseline severity of smell loss correlated with lower nasal prostaglandin E2 levels. Dupilumab increased nasal prostaglandin E2 and decreased levels of nasal albumin, nasal and urinary leukotriene E4, and serum and nasal IgE. Transcripts related to epithelial dysfunction and leukocyte activation and migration were downregulated in inferior turbinate tissue after treatment with dupilumab. There were no dupilumab-induced changes in markers of nasal eosinophilia. We conclude that inhibition of IL-4Ra in AERD led to rapid improvement in respiratory symptoms and smell, with a concomitant improvement in epithelial barrier function, a decrease in inflammatory eicosanoid levels, and an increase in the anti-inflammatory eicosanoid prostaglandin E2. The therapeutic effects of dupilumab are likely due to decreased IL-4Ra signaling on respiratory tissue granulocytes, epithelial cells, and B cells.
Loss of smell in patients with aspirin-exacerbated respiratory disease impacts mental health and quality of life
Raffi Tchekmedyian, Marie Lundberg, Kathleen M Buchheit, Rie Maurer, Deborah Gakpo, Jyotsna Mullur, Jillian C Bensko, Tanya M Laidlaw. Clin Exp Allergy. 2022 May 3.1414-1421 doi: 10.1111/cea.14157 PMID: 35506180. PMCID: PMC9630163
A common problem for AERD patients is loss of sense of smell, or anosmia, but its impact on patients’ quality of life, mental health, and physical wellbeing has been poorly studied. We developed a new questionnaire about the consequences of anosmia, which, along with several other quality-of-life questionnaires was sent to all our Registry patients. Eighty-five percent of the 853 patients who answered the questionnaires (Thank you!) reported diminished sense of smell and/or taste, and we learned that their loss of smell severely impacts their physical, emotional, and mental health. Many patients with diminished smell responded that they could not identify spoiled food (86%), did not enjoy food (71%), felt unsafe (63%), and had encountered dangerous situations (51%) because of their poor smell.
We think that the importance of sense of smell and the relevance of anosmia to patients’ lives should be acknowledged and evaluated by clinicians caring for these patients.
Influence of daily aspirin therapy on ACE2 expression and function-implications for SARS-CoV-2 and patients with aspirin-exacerbated respiratory disease
Buchheit KM, Hacker JJ, Gakpo DH, Mullur J, Sohail A, Laidlaw TM. Clin Exp Allergy. 2021 Jul;51(7):968-971. doi: 10.1111/cea.13898. PMID: 33987897; PMCID: PMC8239915.
In this study, we studied samples of plasma, nasal fluid, and nasal cells from patients with and without AERD or asthma, to determine if 8 weeks of treatment with 650mg aspirin twice daily changed nasal epithelial transcript expression of either ACE2 or TMPRSS2 or altered levels of angiotensin-derived peptides that may be relevant to survival of acute respiratory distress syndrome. We found that the high-dose aspirin treatment did not have any significant effect on any of these biomarkers. Furthermore, consistent with studies showing asthma is generally not a risk factor for severe COVID-19, our data show that asthmatic subjects did not have any difference in angiotensin-derived peptide levels compared to controls. Although we are not able to extrapolate these results out for patients who are on aspirin for more than eight weeks, our data suggest that there is no immunologic indication that daily aspirin therapy would lead to an increased susceptibility to more severe COVID-19.
Dupilumab as an adjunct to surgery in patients with aspirin-exacerbated respiratory disease
Patel P, Bensko JC, Bhattacharyya N, Laidlaw TM, Buchheit KM. Ann Allergy Asthma Immunol. 2022 Mar;128(3):326-328. doi: 10.1016/j.anai.2021.11.020. PMID: 34863953; PMCID: PMC8882131.
We report a series of eight patients with AERD who had experienced a rapid recurrence of nasal polyps after a prior endoscopic sinus surgery, and who subsequently went on to have a revision endoscopic sinus surgery with perioperative initiation of dupilumab, a monoclonal antibody targeting interleukin 4Ra. We found that dupilumab led to no or slower nasal polyp recurrence after surgery compared to the prior sinus surgery, and perioperative initiation of dupilumab therapy also improved upper airway symptoms. This study provides evidence for the use of dupilumab as an adjunct to surgery to prevent polyp regrowth for patients with AERD and insufficient response to standard-of-care therapies.
Dupilumab-associated arthralgia in patients with aspirin-exacerbated respiratory disease
Miss Ozuna L, Ryan T, Bensko JC, Laidlaw TM, Buchheit KM. Ann Allergy Asthma Immunol. 2022 Apr;128(4):469-472. doi: 10.1016/j.anai.2022.01.036. PMID: 35124224; PMCID: PMC8977235.
We report a series of eight patients with AERD who developed arthralgias after initiating dupilumab. We found that 8 patients out of the 160 (5%) with AERD who have been prescribed dupilumab at our center developed possible dupilumab-associated arthralgias. Of the patients who underwent extensive rheumatologic work-up, no etiology of the joint symptoms was identified. All 8 patients experienced significant improvement in upper and lower respiratory tract symptoms and ultimately decided to continue dupilumab even with the possible dupilumab-associated arthralgias. Out of the eight subjects, the arthralgias spontaneously resolved in five of the patients. The cause of the dupilumab-associated arthralgias is unknown, but one possible explanation is a Type 1 or Type 3 “escape” with inhibition of IL-4Ra.
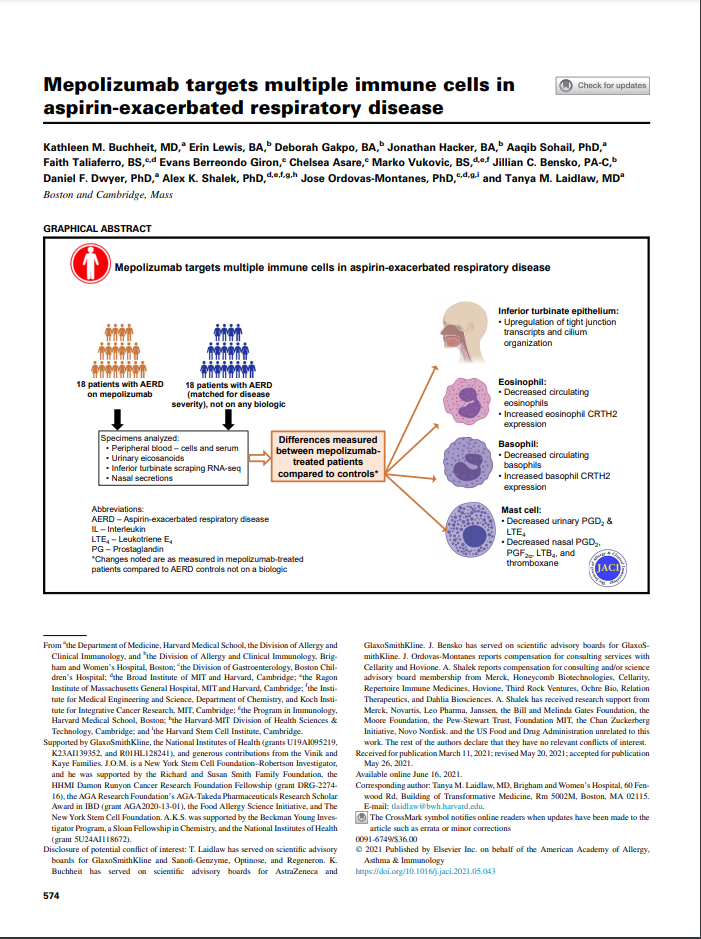
Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease
Buchheit KM, Lewis E, Gakpo D, Hacker J, Sohail A, Taliaferro F, Berreondo Giron E, Asare C, Vukovic M, Bensko JC, Dwyer DF, Shalek AK, Ordovas-Montanes J, Laidlaw TM. J Allergy Clin Immunol. 2021 Aug;148(2):574-584. doi: 10.1016/j.jaci.2021.05.043. PMID: 34144111; PMCID: PMC9096876.
To identify the mechanisms by which anti-IL-5 treatment improves respiratory inflammation in AERD, we studied 18 patients with AERD on mepolizumab for severe eosinophilic asthma compared to 18 matched control patients with AERD not on mepolizumab. Subjects with AERD treated with mepolizumab, had decreased production of inflammatory eicosanoids, and upregulation of nasal epithelial cell transcripts involved in tight junction pathways and cilium organization, compared to AERD patients not treated with mepolizumab. These effects of mepolizumab are likely due to decreased signaling of IL-5 on local respiratory tissue eosinophils, basophils, mast cells, and epithelial cells – all of which have functional IL-5Rα. We determined that the mechanism by which IL-5 inhibition provides therapeutic benefit in respiratory inflammation is not due exclusively to anti-eosinophil effects.
Safety, outcomes, and recommendations for two-step outpatient nonsteroidal anti-inflammatory drug challenges
Li L, Bensko J, Buchheit K, Saff RR, Laidlaw TM. J Allergy Clin Immunol Pract. 2022 May;10(5):1286-1292.e2. doi: 10.1016/j.jaip.2021.11.006. PMID: 34800703; PMCID: PMC9086081.
Drug challenge protocols for evaluation of non-AERD related NSAID hypersensitivity can vary widely by provider and institution and can be prohibitively time and resource intensive to perform. In this cohort of 249 individuals undergoing NSAID drug challenges, we describe the safety and outcomes of a 2-step NSAID drug challenge protocol. Over 85% of challenges were negative for an immediate or delayed reaction, allowing patients to use at least one clinically indicated NSAID. Challenge reactions were generally mild. These results indicate that a simple 2-step NSAID challenge protocol may be safely used in the outpatient setting to delabel appropriate candidates.
Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Rα in a real-world setting
Bavaro N, Gakpo D, Mittal A, Bensko JC, Laidlaw TM, Buchheit KM. J Allergy Clin Immunol Pract. 2021 Feb 22:S2213-2198(21)00200-2. doi: 10.1016/j.jaip.2021.02.020. PMID: 33631410; PMCID: PMC8277677
In recent years, biologics have become a more common treatment option for patients with aspirin-exacerbated respiratory disease (AERD). Respiratory biologic medications targeting interleukin (IL)-4Ra, IL-5, IL-5Ra, and IgE are approved for severe asthma and have recently been approved or being studied for CRSwNP. Treatment with biologic medications targeting type 2 cytokines and cytokine receptors is an attractive option for treating AERD, but data guiding selection of the appropriate agent are limited. In this study, we examined 41 patients who were treated with mepolizumab, reslizumab, or benralizumab. 27 of the 41 patients transitioned to dupilumab for inadequately controlled symptoms while 14 remained on mepolizumab.
For the 27 who switched to dupilumab, their SNOT-22 and ACT scores, and the FEV1% predicted did not improve while they were on the anti-IL-5/IL-5Ra biologic. However, there was significant improvement in symptoms scores and lung function, and reduction in asthma exacerbations while on dupilumab. For the 14 patients that remained on mepolizumab, they saw a significant improvement in total SNOT-22 and ACT scores but not in lung function. Of note, the only difference between the two groups was the baseline IgE level, which may serve as a useful biomarker for biologic selection in AERD patients. Although the study is limited by the retrospective nature of the data, it highlights the need for head-to-head, prospective studies aimed at understanding identifying responder endotypes to guide selection of appropriate biologic therapy.
Aspirin-Exacerbated Respiratory Disease: Association Between Patient-Reported Sinus and Asthma Morbidity
Bergmark RW, Palumbo M, Rahman S, Maurer R, Dominas C, Roditi R, Bhattacharyya N, Maxfield A, Buchheit KM, Laidlaw TM. J Allergy Clin Immunol Pract. 2020 Dec 8:S2213-2198(20)31278-2. doi: 10.1016/j.jaip.2020.11.051.PMID: 33307278.
This study looked to establish and understand the connection between pulmonary and sinonasal symptoms in aspirin-exacerbated respiratory disease (AERD). To better understand the connection, the investigators reviewed the Sino-Nasal Outcomes Test (SNOT) 22-item and Asthma Control Test (ACT) scores, and percent predicted FEV1 (FEV1% predicted) from 1065 AERD Registry participants. The SNOT-22 was used as the predictor variable while ACT score and FEV1% predicted served as primary outcomes. Any ten-point increase in SNOT-22 was associated with a 0.87-point decrease in ACT and a 0.75% decrease in FEV1%. Any one-point increase in ACT was associated with a 1.0% increase in FEV1% This study also demonstrates an association between subjective and objective measures of asthma severity.
.
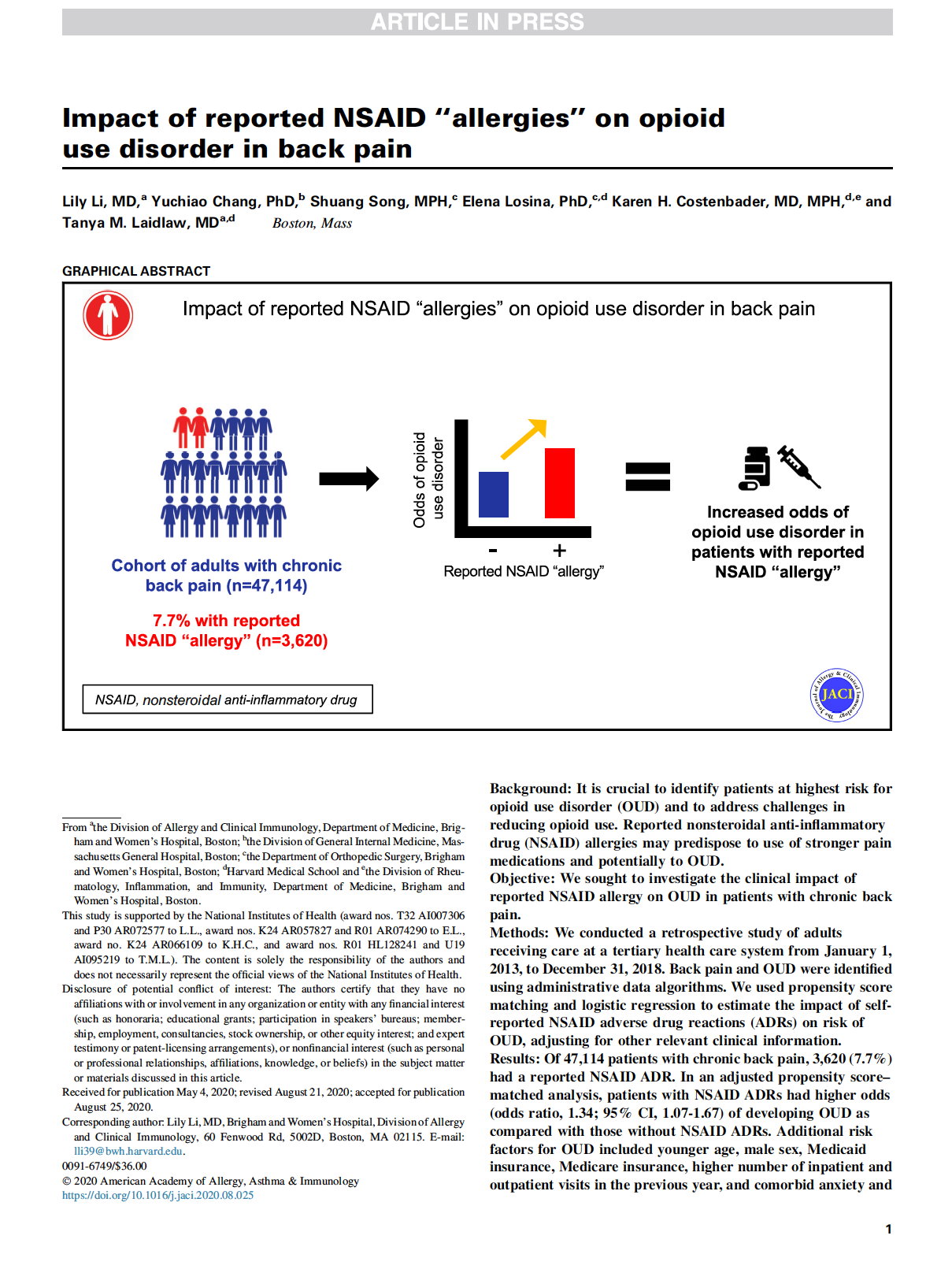
Impact of reported NSAID “allergies” on opioid use disorder in back pain
Li L, Chang Y, Song S, Losina E, Costenbader KH, Laidlaw TM. J Allergy Clin Immunol. 2020 Sep 9:S0091-6749(20)31236-7. doi: 10.1016/j.jaci.2020.08.025. PMID: 32916184; PMCID: PMC7995999.
Li et al. investigated the clinical impact of reported nonsteroidal anti-inflammatory drug (NSAID) allergy on opioid use disorder (OUD) in patients with chronic back pain. NSAIDs are commonly prescribed for treatment of acute and chronic pain, but their therapeutic use is limited by reported allergies. Allergy overreporting and lack of further investigation can lead to unnecessary avoidance of NSAIDs and increased utilization of alternative analgesics, including opioids, to manage pain. The investigators looked at over 47,000 patients with chronic back pain of whom over 3,600 (7.7%) had an NSAID allergy listed in their health record. They found that patients with chronic back pain and self-reported NSAID allergies are at increased risk of being prescribed opioids and developing OUD. Patients with chronic back pain and NSAID allergies should be referred to allergy specialists for potential allergy delabeling in order to minimize unnecessary opioid prescribing and to reduce the risk of OUD.
IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease
Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, Lai J, Bankova LG, Bhattacharyya N, Shalek AK, Barrett NA, Boyce JA, Laidlaw TM. J Allergy Clin Immunol. 2020 June;145(6):1574-1584. doi: 10.1016/j.jaci.2020.02.035. PMID: 32199912;PMCID: PMC7282948.
This study showed that sinus tissue IgE and IgG4 levels were elevated in patients with AERD compared to controls and that subjects with AERD whose nasal polyps recurred rapidly had higher IgE levels than did subjects with AERD, with slower polyp regrowth. Single-cell RNA sequencing revealed increased IL5RA, IGHG4, and IGHE in antibody-expressing cells from patients with AERD compared to those from NSAID-tolerant patients with CRSwNP, and there were more IL-5Rα+plasma cells in the polyp tissue from AERD than in polyp tissue from CRSwNP. IL-5 stimulation of plasma cells in vitro induced changes in a distinct set of transcripts. These findings suggest a role for IL-5Rα+ antibody-expressing cells in facilitating local antibody production and severe nasal polyps in AERD.
Unique Effect of Aspirin Therapy on Biomarkers in Aspirin-exacerbated Respiratory Disease. A Prospective Trial
Cahill KN, Cui J, Kothari P, Murphy K, Raby BA, Singer J, Israel E, Boyce JA, Laidlaw TM. Am J Resp Crit Care Med 2019 Sept;200(6):704-711. doi: 10.1164/rccm.201809-1755OC. PMID:30978291;PMCID: PMC6775876
These analyses revealed that 8 weeks of treatment with high-dose aspirin therapy decreased nasal symptoms and urinary prostaglandin E metabolite and increased urinary leukotriene E4 levels in subjects with AERD, but not in those with aspirin-tolerant asthma. Only in subjects with AERD, exhaled nitric oxide, plasma tryptase, and blood eosinophil and basophil counts increased on aspirin. These findings show that high-dose aspirin therapy for 8 weeks paradoxically increases markers of type 2 inflammation in AERD, despite reducing nasal symptoms. This effect of aspirin is unique to AERD and not observed in subjects with aspirin-tolerant asthma.
Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD
Laidlaw TM, Mullol J, Fan C, Zhang D, Amin N, Khan A, Chao J, Mannent LP. J Allergy Clin Immunol Pract. 2019 Sep-Oct;7(7):2462-2465.e1. doi: 10.1016/j.jaip.2019.03.044. PMID:30954643
In this analysis of Phase 2 data of the efficacy of dupilumab in treating patients with CRSwNP, we found that in the subgroup of patients with CRSwNP and comorbid AERD, dupilumab significantly improved CRSwNP disease outcomes, along with asthma control and lung function. In some cases, the dupilumab-induced improvement in outcomes was greater in the patients with AERD than it was in the aspirin-tolerant CRSwNP patients.
Sinus surgery improves lower respiratory tract reactivity during aspirin desensitization for AERD
Huang GX, Palumbo ML, Singer JI, Cahill KN, Laidlaw TM. J Allergy Clin Immunol Pract. 2019. May-Jun;77(5):1647-1649.doi: 10.1016/j.jaip.2019.02.037. PMID:30877073; PMCID: PMC7110416
This retrospective clinical study found that for patients with AERD, when a recent endoscopic sinus surgery precedes aspirin challenge or aspirin desensitization, less aspirin-induced lower airway reactivity and improved safety are observed.
Plasma tryptase elevation during aspirin-induced reactions in aspirin-exacerbated respiratory disease
Cahill KN, Murphy K, Singer J, Israel E, Boyce JA, Laidlaw TM. P J Allergy Clin Immunol. 2019 Feb;143(2):799-803.e2. doi: 10.1016/j.jaci.2018.10.007.PMID: 30339852;PMCID: PMC6519056
This study of patients with AERD found that an elevation of plasma tryptase during aspirin-induced respiratory reactions occurs in 50% of reactions despite CysLT1R antagonist prophylaxis and identifies lung function decline and extra-respiratory symptoms in AERD.
Dietary Fatty Acid Modification for the Treatment of Aspirin-Exacerbated Respiratory Disease: A Prospective Pilot Trial
Schneider TR, Johns CB, Palumbo ML, Murphy KC, Cahill KN, Laidlaw TM. J Allergy Clin Immunol Pract. 2018 May-June;6(3)825-31. doi10.1016/j.jaip.2017.10.011.PMID:29133219; PMCID: PMC5945343
We followed 10 patients with AERD who completed a 2-week strict dietary intervention that aimed to reduce dietary omega-6 fatty acid consumption to less than 4 grams per day and increase omega-3 intake to more than 3 grams per day. We noted that the dietary change induced a significant decrease in urinary levels of leukotriene E4 and prostaglandin D2 metabolites, and also improved patient symptoms, with a 15-point reduction in the SNOT-22 score and an 0.27-point improvement in the Asthma Control Questionnaire. Therefore we concluded that a high omega-3/low omega-6 diet may be an appropriate adjunct treatment option for patients with AERD.
Allergic inflammatory memory in human respiratory epithelial progenitor cells
Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth MH, Hughes TK, Kazer SW, Yoshimoto E, Cahill KN, Bhattacharyya N, Katz HR, Laidlaw TM, Boyce JA, Barrett NA, Shalek AK. 2018. Aug;560(7720):649-54. doi: 10.1038/s41586-018-0449-8.PMID: 30135581; PMCID: PMC6133715
By profiling human surgical nasal polyp and sinusitis samples, we determined that cultured basal cells retain intrinsic memory of IL-4/IL-13 exposure. Overall this study found that reduced epithelial diversity stemming from functional shifts in basal cells is a key characteristic of type 2 immune-mediated barrier tissue dysfunction. Our results demonstrate that epithelial stem cells may contribute to the persistence of human disease by serving as repositories for allergic memories.
Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size
Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, Lane AP, Palumbo ML, Sullivan M, Archibald D, Dworetzky SI, Hebrank GT, Bozik ME. Laryngoscope. 2019 Feb;129(2)E61-E66.doi: 10.1002/lary.27564. PMID:30284267
This was an open-label study of 16 subjects with CRSwNP, peripheral eosinophilia, and histologic finding of eosinophilic inflammation within their nasal polyp tissue, who were treated for 6 months with the novel eosinophil-lowering agent, dexpramipexole. After 6 months of dexpramipexole, which was quite well tolerated, there was a 94% reduction in blood eosinophils, and a 97% reduction in nasal polyp tissue eosinophils. However, neither the total polyp score, nor any other clinical endpoints significantly improved. This “negative” study has in fact had a dramatic effect on the previous dogma in the field, which suggested that the respiratory tissue eosinophils were a driving cause of patient polyp burden and symptoms. Instead, we found that despite the near-elimination of polyp eosinophils, there was no improvement in symptoms or polyp burden noted.
A trial of type 12 purinergic (P2Y 12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease
Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, Kothari P, Raby B, Israel E, Boyce JA. J Allergy Clin Immunol. 2019 Jan;143(1):316-324.doi: 10.1016/j.jaci.2018.06.001.PMID: 29890239; PMCID: PMC6286686
In this trial, 40 patients with AERD completed a 10-week, double-blind, placebo-controlled crossover trial of prasugrel, and then underwent oral aspirin challenges after 4 weeks of prasugrel and after 4 weeks of placebo. Prasugrel did not significantly change the severity of aspirin-induced reactions or the provocative dose of aspirin. However, 5 subjects (responders) reacted to aspirin while receiving placebo but did not have any reaction to aspirin challenge after the prasugrel arm. Those 5 patients did not have significant aspirin-induced increases in urinary leukotriene E4, prostaglandin D2 metabolite, or thromboxane B2 levels; and did not display increases in serum tryptase levels during aspirin reactions on the placebo arm, all of which were observed in the nonresponders. Therefore, in a small subset of patients with AERD, P2Y12 receptor antagonism with prasugrel completely inhibited all aspirin-induced reaction symptoms, suggesting a contribution from P2Y12 receptor signaling in this subset.
KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma
Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, Garofolo D, Castro M, Jarjour N, DiMango E, Erzurum S, Trevor JL, Shenoy K, Chinchilli VM, Wechsler ME, Laidlaw TM, Boyce JA, Israel, E. N Engl J Med. 2017, 376(20):1911-20. doi: 10.1056/NEJMoa1613125. PMID:28514613; PMCID: PMC5568669
In a randomized, placebo-controlled, 24-week trial of imatinib in patients with poorly controlled severe asthma who had airway hyperresponsiveness despite receiving maximal medical therapy, imatinib decreased airway hyperresponsiveness, mast-cell counts, and tryptase release. These results suggest that KIT-dependent processes and mast cells contribute to the pathobiologic basis of severe asthma.
Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease
Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, Lai J, Bhattacharyya N, Israel E, Boyce JA, and Laidlaw TM. J Allergy Clin Immunol. 2016 May:137(5):1566-1576. doi: 10.1016/j.jaci.2015.10.020. PMID:26691435; PMCID: PMC4860132
This translational study found that urinary levels of a prostaglandin D2 metabolite (uPGD-M) were 2-fold higher in patients with AERD relative to those in aspirin-tolerant subjects and increased further during aspirin-induced reactions. Peak uPGD-M levels during aspirin reactions correlated with reductions in blood eosinophil counts and lung function and increases in nasal congestion. Nasal polyp mast cells expressed high levels of PGD2 synthase, which was correlated with nasal polyp TSLP mRNA expression. Levels of the active form of TSLP were increased in nasal polyps from patients with AERD, and recombinant TSLP induced PGD2 generation by cultured human mast cells. These findings suggested that mast cell-derived PGD2 is a major effector of type 2 immune responses driven by TSLP and that dysregulation of this innate system contributes significantly to the pathophysiology of AERD.
Prostaglandin D₂: a dominant mediator of aspirin-exacerbated respiratory disease
Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. J Allergy Clin Immunol. 2015; 135(1):245-252. doi: 10.1016/j.jaci.2014.07.031. PMID:25218285; PMCID: PMC4289104
This observational study stratified 29 patients with AERD into those who tolerated aspirin desensitization and those who did not. Urine was analyzed for eicosanoid metabolites at baseline, during aspirin reactions, and during high-dose aspirin therapy. Blood was analyzed for cell differentials at baseline and during aspirin therapy. We found that failure to tolerate aspirin desensitization, and the development of severe extrapulmonary symptoms during the aspirin-induced reactions in AERD, is associated with prostaglandin D2 overproduction.
Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes
Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, Castells MC, Chhay H, Boyce JA. Blood. 2012 Apr 19;119(16):3790-8. doi: 10.1182/blood-2011-10-384826.PMID: 22262771; PMCID: PMC3335383.
This study found that nasal polyps from subjects with AERD contained many extravascular platelets that colocalized with leukocytes, and the percentages of circulating neutrophils, eosinophils, and monocytes with adherent platelets were higher in the blood of subjects with AERD than in aspirin-tolerant controls. Platelet-adherent subsets of leukocytes had higher expression of several adhesion markers than did platelet nonadherent subsets. Adherent platelets contributed much of the LTC4 synthase activity of peripheral blood granulocytes, and urinary LTE4 levels correlated with percentages of circulating platelet-adherent granulocytes. Because platelet adherence to leukocytes allows for augmented transcellular conversion of leukotrienes, a disturbance in platelet-leukocyte interactions may be partly responsible for the overproduction of cysteinyl leukotrienes that characterize AERD.
Characterization of a novel human mast cell line that responds to stem cell factor and expresses functional FcεRI
Laidlaw TM, Steinke JW, Tiñana AM, Feng C, Xing W, Lam BK, Paruchuri S, Boyce JA, Borish L. J Allergy Clin Immunol. 2011; 127:815-22.doi: 10.1016/j.jaci.2010.12.1101.PMID:21281958; PMCID: PMC3052637
This work characterized a human MC line, termed “LUVA cells” that arose spontaneously from a culture of nontransformed hematopoietic progenitor cells. LUVA cells are an immortalized human MC line that can be maintained without stem cell factor and display high levels of normally signaling c-kit and FcεRI, and may be valuable for future functional human MC studies.